|
IT AIN’T NATURAL: Was The Pandemic The Result of Batty Experiments? By A. Kronstadt [Scientific issues are very easy to obfuscate because few people understand the details. The origins of the Covid-19 pandemic will remain controversial because the basic principles of science involved are not universally understood. Do you know what a receptor molecule is, or that protein molecules are composed of amino acids, which are in turn coded by DNA molecules? I have made an effort to explain some of these things in passing, and I hope that I’ve done a good job in that respect. The information that I cite below will not convince everyone but it has convinced me that irresponsible science, not necessarily biological warfare but ultimately its equivalent, brought about the Covid-19 plague. The most essential concept to understand here is that of the gain of function (GoF) experiment, which we will detail here.]
Most of the narratives concerning the origins of the Covid-19 pandemic center on the city of Wuhan in central China and on the Chinese rufous horseshoe bat (Rhinolophus sinicus), a sparrow-sized, fruit- and insect-eating bat that was found by Chinese researchers in 2013 to be a reservoir for the virus SARSr-CoV, then recognized to be a close relative of the original SARS virus that had been spreading in 2002-2004. That virus had been associated with civets (mongoose-like animals) that were sold in the marketplaces of south China including the large metropolis of Guangzhou; it produced symptoms similar to those of Covid-19 but did not spread as rapidly. When Covid-19’s virus was recognized in 2020, the previous SARS virus was designated SARS-CoV-1 and the agent of the 2020 Covid-19 pandemic as SARS-CoV-2.
The CDC and most of mainstream science tells an official story whereby a natural bat-to-human transmission, followed by an adaptation of the virus to human-to-human transmission, was the origin of Covid-19. However, the rufous horseshoe bat has only been found to be the natural reservoir of a virus resembling SARS-CoV-1 (agent of the 2002-2004 outbreak); no virus that could be construed as being close to SARS-CoV-2, the agent of Covid-19, has ever been isolated from a wild bat. The official story is therefore an extrapolation from research involving a different virus, and from the fact that rufous horseshoe bats and other bats actually do harbor many different coronaviruses.
The first cases of Covid-19 were observed in Wuhan in late 2019, and the official story has it that they resulted from the virus first having been transmitted from bats to humans and then having acquired the ability to transmit from humans to humans. The IUCN Red List of Endangered Species (2018) has provided a map [predating the pandemic and the notoriety conferred on the bats and Wuhan] that conveniently juxtaposes the location of Wuhan with the range of the Chinese rufous horseshoe bat.
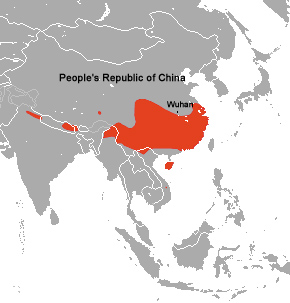 Range of the Chinese Rufous Horseshoe Bat
We can see from this that Wuhan is not at the center of the range of this species of bat but just north of its northern limit. Rhinolophus sinicus, like the SARS-CoV-1 virus that it carries, is associated with the south of China while Wuhan is located in the center of that nation. The bats that were originally found to harbor SARSr-CoV (aka SARS-CoV-1) in the above mentioned 2013 study were sampled near Kunming in southern Yunnan province, 802 miles from Wuhan (see map). It had been reported that in 2012, six miners fell ill from a SARS-like illness in Yunnan province while working in a mine filled with bat feces. The actual strain of coronavirus involved was not determined and no human-to-human transmission was assumed.
Very early in the timeline of the pandemic, in December 2019 , eight days after Beijing acknowledged the existence of a new virus, Chinese officials banned the sale of live animals in the public marketplaces of Enshi prefecture, an area 334 miles west of Wuhan where there are many caves harboring Rhinolophus sinicus. In 2020, WHO officials wanting to investigate the origins of Covid-19 were denied access to these caves by the government. The interesting thing here is not so much that the Chinese government seems to have suspected bats as the culprits--but that the suspect bats were 334 miles away from Wuhan.
Wuhan itself has no wild Rhinolophus sinicus in its immediate environs, and was not a likely place for either bat-to-human or human-to-human transmission of the coronavirus to have evolved. However this is not to say that the animal was not present in the city. Science had brought both the rufous horseshoe bat and its pathogens to Wuhan in order to experiment with them. Many of the individual facts that we will bring out here are actually old news, but when viewed all in one place present an irrefutable argument that (1) there was no natural bat-to-human transmission event that resulted in the creation of SARS-CoV, the agent of the Covid-19 pandemic, and (2) the disease emerged in an already highly human-infectious form, inconsistent with the usual course of viral evolution in which a weakly human-infectious virus becomes more infectious to humans via natural selection while passing through the human population.
Virus Basics Viruses are not living things but are usually thought of either as offshoots of living matter or as relics of the very early history of living matter. They are always associated with a “host” from the world of living things, which may be something as simple as a bacterium or as complex as a human or other mammal. There are millions of different viruses, which only affect one, or a very limited range of host species.
A virus consists of a nucleic acid part and a protein part. Nucleic acids, which may take the form of RNA or DNA, are large molecules with a lot of diversity that contain a code for producing proteins, the main structural elements of both the virus and the host. Proteins also constitute the enzymes that determine what chemical reactions an organism can carry out, and are thereby the backbone of life itself. The host has thousands of proteins that it needs in order to carry out its own metabolism, each of them coded by a stretch of nucleic acid (always DNA for higher organisms). A virus has no metabolism of its own, but its RNA or DNA can hack into the existing DNA of the host and exploit its metabolic mechanisms to produce the proteins of the virus, and thereby more viral particles which can spread through the host and move on to additional hosts.
The virus therefore harms the host by draining off its nutrients, clogging up its cells with viral proteins, causing the death of the cells by breaking their membranes, and especially in the case of Covid-19 by provoking a violent immune reaction that does more damage to the host than the virus itself.
All of the coronaviruses including SARS-CoV-1 and SARS-CoV-2 have RNA (ribonucleic acid) as their nucleic acid part, which codes for the proteins that the virus will force the cells of the host to produce. All of the hosts--be they humans, bats, or even plants--use DNA (deoxyribonucleic acid) to store their genetic information. DNA is a more stable, complex form of nucleic acid which is found all genuine living things; only certain viruses use RNA, while other viruses use DNA like their hosts. RNA is unstable and mutates more than DNA. This is why the coronaviruses are continually evolving “variants” with different proteins. Since a vaccine works by targeting specific proteins, it is necessary to continually update vaccines against RNA viruses to go after the new mutant proteins. This has been the case with the SARS-CoV-2 vaccines, whose effectiveness has dropped dramatically even in the year that they have been available, and also with vaccines against the influenza virus, which is likewise an RNA virus, where vaccine effectiveness is often less than 50%. However, the smallpox virus is a DNA virus, with much more stable reproduction of its genetic material, and that is the reason why it has been possible to immunize people against the same strain of virus since the 19th century with essentially 100% effectiveness.
In order to infect a host cell, the virus must find a door to come through, or better put, because a virus cannot move on its own, it must find a conveyor belt provided by the cell itself. It must attach itself to a host protein on the surface of the cell called a receptor, which the cell normally uses to bring in useful proteins such as its own nutrients. A pathogenic virus has a fake protein part that fools the cell into believing that it is the protein that it wants to bring in. Humans and other mammals have receptors for an enzyme called angiotensin-converting converting enzyme 2 (ACE2) on the surface of many of their cells, which plays a not completely understood role in regulating blood pressure. Coronaviruses such as those in the SARS family (SARS-CoV-1 and -2) have adopted the ACE2 receptor as their own receptor, or entry point, into the cells of mammals. This has happened because of purely evolutionary, opportunistic considerations having nothing to do with the original function of ACE2 or its receptor. The spike protein which makes up the outermost projections on the surface of coronaviruses fits into the structure of the ACE2 receptor like a key in a lock (perhaps less perfectly but to varying extents depending on the variant) because of its own structure or “folding” pattern.
It is important for anyone
studying Covid-19 to understand the concept of a
receptor and particularly the ACE2 receptor, because
the agent of Covid-19, SARS-CoV-2, had enormous
affinity for the human version of this receptor even
in the very first cases of Covid-19 that appeared
early in 2020. In most cases, a virus must pass
through thousands of hosts in order to evolve this
level of affinity for a receptor. The SARS-CoV-1
virus, agent of the 2002-2004 SARS outbreak, only
showed a fraction of this level of infectivity when it
first appeared. Furthermore, SARS-CoV-2 also has a
structure in its spike protein called a furin-cleavage
site which is activated by an enzyme in the host
respiratory epithelium called furin, enabling it to
react with yet another receptor specifically on the
surface of the cells of the human respiratory
epithelium, Neuropilin-1. SARS-CoV-1, the agent of the
2002-2004 SARS epidemic, does not have a
furin-cleavage site, nor does any bat coronavirus that
has been investigated as a relative of SARS-CoV-2.
There is reason to believe that the enhanced affinity
for the ACE2 receptor as well as the presence of the
furin-cleavage site are the products of genetic
engineering in a gain-of-function experiment, as these
features of the Covid-19 virus adapt it very
specifically to infecting the human respiratory system
through aerosols, something that SARS-CoV-1 is unable
to do. What Is A Gain-of Function (GoF) Experiment? In the broader sense of the term, a gain-of-function experiment is an experiment in which a gene is either added and/or activated so that the cell produces a protein that it did not produce before. By the same token, a loss-of-function experiment is one in which a gene is removed or inactivated such that a certain protein is no longer produced. These are two basic procedures in genetic engineering. An older example of a gain-of function experiment defined in this broad way is to add the gene for insulin from a human to yeast or bacterial cells, making them mass produce insulin, which was an early, highly successful application of genetic engineering to the pharmaceutical industry.
However, over the course of the 21st century “gain-of-function” as applied to viral disease research has meant gain of the disease-producing function, which typically might involve manipulation of the receptor-binding part of a protein to produce a mutant protein that interacts with a different receptor molecule and thereby is able to infect a different host, or the same host more efficiently. Why would researchers want to make a virus more versatile or efficient, i.e., whose side are they on, us or the virus?
The stated reason is experimental: they ostensibly want to anticipate the evolutionary development of the virus in order to develop a vaccine or drug to combat it. Keep in mind though that (1) evolution will take its own course and there is no reason to believe that the mutations introduced in the lab will necessarily correspond to those that are brewing in nature, and (2) the GoF experiment will have brought a pathogen into the world that did not exist before. Scientists are accused of playing God, but with some of these GoF experiments they are really playing Satan, and, as we will see, the devil is in the details.
Playing Cat and Mouse With Nature As we have stated above, the spike protein of coronaviruses is the one that interacts with receptors on the host cell (receptor-binding domain or RBD). At Utrecht University in the Netherlands in 1999, researchers swapped the receptor binding domain of a coronavirus specific to cats with that of a coronavirus specific to mice, and vice versa. They then tested each of the viruses in tissue culture with cat and mouse cells, respectively, noting that the tropism, or specificity of the RBDs for animal cells of a particular species, had been reversed. This was the first and probably least disturbing of the many GoF experiments involving the spike proteins of various coronaviruses.
The feline coronavirus (FCoV) is present in 20% to 60% of the cat population. It is more common in cats that spent part of their lives housed in indoor shelters than it is in either completely feral cats or or in house cats. Generally FCoV is associated with mild gastrointestinal symptoms in cats, because the virus has an attraction or ‘tropism’ for receptors present in the intestine. However, in a relatively small percentage of infected cats, FCoV undergoes a spontaneous mutation that changes the tropism of the spike protein “binding domain” to attach to a receptor on the animal’s macrophages, a fundamental type of immune cell necessary for disease resistance. The viruses that have mutated are no longer called FCoV, but feline infectious peritonitis virus (FIPV), and cause a inflammation of the mucus membranes lining the cat’s abdominal cavity, resulting either in the accumulation of massive amounts of fluid or the spread of deadly tumors called granulomas, made up of abnormal macrophages, through the animal’s abdominal cavity, resulting in painful death within a few days or weeks.
Because the spontaneous transition between the less pathogenic FCoV and the deadly FIPV is an example of a natural “gain of function” event, this virus was one of the first to attract the attention of researchers interested in manipulating gain of function as a research tool for establishing how a virus at first nonpathogenic for a particular species can become pathogenic.
The first such experiment was performed in 2000 at the David Axelrod Institute, which is part of the Wadsworth Center in Albany, a vast research complex maintained by the New York State Department of Health. In the abstract of their article, Kuo and colleagues summarize their work like so: “Using targeted RNA recombination, we constructed a mutant of the coronavirus mouse hepatitis virus (MHV) in which the ectodomain of the spike glycoprotein (S) was replaced with the highly divergent ectodomain of the S protein of feline infectious peritonitis virus. The resulting chimeric virus, designated fMHV, acquired the ability to infect feline cells and simultaneously lost the ability to infect murine cells in tissue culture. “
Their work was reproduced in the Netherlands in 2003 by Bert Jan Haijema, Haukeliene Volders, and Peter J. M. Rottier: “We first constructed by targeted recombination a mutant of FIPV, designated mFIPV, in which the ectodomain of the spike glycoprotein was replaced by that of MHV. This switch allowed for the selection of the recombinant virus in murine cells: mFIPV grows to high titers in these cells but has lost the ability to grow in feline cells. In a second, reverse process, mFIPV was used as the recipient, and the reintroduction of the FIPV spike now allowed for selection of candidate recombinants by their regained ability to grow in feline cells.”
Haijema et al. determined the virulence of their recombinant mouse-to-cat virus by inoculating it oronasally into nine 5-month-old kittens, three out of four of which were euthanized due to advanced symptoms of feline infectious peritonitis between weeks 2 and 6 after inoculation. As an aside, the victims of this cruel experiment were “healthy, female, specific-pathogen-free HsdCpd:CAD(BR) kittens” (meaning that they were inbred and raised in a germ-free bubble) supplied by Harlan Sprague-Dawley, Inc., a company repeatedly attacked by animal rights activists.
Furin-Cleavage Site: The Maker’s Mark FECV/FIPV is an alphacoronavirus, but the SARS viruses, including the pathogen identified in COVID-19, SARS-CoV-2, are betacoronaviruses. For this reason, nobody suggests that the above GoF experiments were at the origin of SARS-CoV-2. However, these early experiments already raise questions that reflect on the natural versus laboratory origin of SARS-CoV-2. We mention above that the spike protein of SARS-CoV-2 includes a furin-cleavage site which interacts with an enzyme called furin that is found in human respiratory tissues, and that when the furin cleaves this part of the protein, a new receptor is made available to the virus, which is thus enabled to target respiratory tissues in people. Licitra et al. pointed out in their 2013 article in Emerging Infectious Diseases (Mutation in Spike Protein Cleavage Site and Pathogenesis of Feline Coronavirus) that it is a mutation in the furin-cleavage site that results in the transition from the nonpathogenic FECV to the highly infectious FIPV. The furin-cleavage site of FECV, without the mutation, is activated only in the intestinal epithelium, whereas the mutated furin-cleavage site enables the virus to attack macrophages, front-line immune cells that travel through the entire body. It was in these experiments that molecular biologists learned to use a furin-cleavage site as a kind of switch to selectively turn the pathogenicity of a virus on and off depending on the species and tissue. Once again we must remember why gain-of-function experiments are described as “dual-use,” both medical and potentially military: simply substitute the word “trigger” for “switch” in the previous sentence.
In 2011-2012, researchers at the University of Wisconsin-Madison engineered a new strain of influenza H5N1 virus (which is not classed as a coronavirus), the normal strain of which affects birds and occasionally humans, that encoded a gene for the hemagglutinin protein, enabling it to infect the cells of mammals with greater efficiency. H5N1 does not normally transmit via the airborne route, but the laboratory strain of H5N1 was shown to spread between ferrets via the airborne route. The new strain also showed a higher affinity for human lung tissue in tissue culture, but was not tested directly on humans. This experiment, characterized in a New York Times op-ed piece as an “engineered doomsday,” resulted in greater scrutiny of GoF experiments and a voluntary moratorium on such research backed by the CDC and Anthony Fauci, covering only influenza viruses. Looking at Fauci’s own 2012 position paper on the subject (Research on Highly Pathogenic H5N1 Influenza Virus: The Way Forward), one can see that he carefully words the message so that it only covers H5N1. The voluntary pause lasted for only 60 days and was replaced by a set of rules in which the Obama administration actually approved and encourages certain classes of GoF research. All explicit restrictions on GoF research were entirely lifted by the Trump administration in Dec. 2017.
A brief summary is therefore in order before we focus again on the Wuhan Institute of Virology. Many coronaviruses that cause disease in mammals including humans use the ACE2 receptor to enter the cell. This is the case with both SARS-CoV-1, the agent of the 2011-2012 SARS outbreak in South Asia, and its relative SARS-CoV-2, the agent of Covid-19, as well as the less closely related feline coronaviruses FECV and FIPV. The “cat and mouse” gain of function experiments performed early in this century taught molecular biologists to use the furin cleavage site in the spike protein as as a switch or trigger to target coronaviruses from one kind of tissue to another based on the availability of furin in those tissues. SARS-CoV-1, the agent of the 2002 SARS outbreak, does not contain a furin-cleavage site.
What we have made clear so far is that SARS-CoV-2 sprang into the world fully able to pass from human to human with great virulence in a way that would be very atypical assuming a natural adaptation from bat to human. Generally a virus starts out less adapted and becomes more adapted with time. Furthermore, there is no candidate among natural bat viruses for a “missing link” in natural evolution that has even been suggested as the bat precursor of the human-adapted SARS-CoV-2. This last statement of course needs to be qualified by saying that the missing link need not have come from nature in this age of genetic engineering, particularly if ground zero is in the city of Wuhan in proximity to one of the world’s great centers of gain-of-function experimentation.
And thus, here is the main hypothesis of this article. The missing link is somewhere in a freezer at the Wuhan Institute of Virology. If someone were to revive it, it would turn out only to infect bats; the molecular hardware that targets it to humans was added via genetic engineering at the Institute. It was one of a number of bat viruses that were brought to Wuhan and subjected to gain-of-function experimentation supposedly to demonstrate how the bat-to-human transition would or could occur, with the arrogant but noble aim of heading it off with a vaccine, before it even evolved. The money for this experimentation did not come entirely from China but also from the United States via a grant from the National Institutes of Health to something called the EcoHealth Alliance, a driving force in the use of GoF experiments to study emerging viruses.
What Were They Doing At The Wuhan Institute of Virology? In an article by Nicholas Wade in Bulletin of the Atomic Scientists (May 5, 2021), Nobel-prize-winning virologist David Baltimore referred to the furin-cleavage site of SARS-CoV-2 as the “smoking gun” for the laboratory origin of the virus. He later walked this statement back to: “I believe that the question of whether the sequence was put in naturally or by molecular manipulation is very hard to determine but I wouldn’t rule out either origin.”
SARS-CoV-2 also exhibits, if not a smoking gun but at least a molecular fingerprint that identifies it with a gain of function experiment. As Stephen Quay and Richard Muller discuss in their Oct. 5, 2021 article in the Wall Street Journal, part of the way that the spike protein of SARS-CoV-2 differs from that of other coronaviruses including SARS-CoV-1 is that in the amino acid sequence of the SARS-CoV-2 version of the spike protein, there are two copies of the amino acid arginine in a row, whereas in SARS-CoV-1 and other coronaviruses the sequence contains only one arginine. Did the second arginine simply “evolve” naturally in a bat or some other organism, or was it engineered into the spike protein in order to impart a particular feature that a scientist wished to see? Quay and Muller point to the DNA coding for the two arginines—it is “CGG-CGG” (cytosine-guanine-guanine repeated twice). There are actually 36 different ways for DNA to code for two successive arginines, and for both of the arginines to have the same three DNA bases in their “codon” is the least likely way for it to happen. However, in genetic engineering, CGG-CGG would be the most likely way scientists would choose to code for two successive arginines. It can be viewed as a stitch or seam that indicates two things have been sewed together.
The Wuhan-America Connection The secret as to how this happened resides with three individuals who planned the gain of function experiments with bat coronaviruses at the Wuhan Institute of Virology: Peter Daszak, Ralph Baric, and Zhi Zhengli. The notorious “bat-man” gain-of-function did not begin in China, but at the University of North Carolina at Chapel Hill in the laboratory of Ralph Baric, where “reverse genetics” experiments were conducted in 2013-2015 to transfer the gene encoding the spike protein from a novel coronavirus, SHC014, into the genome of SARS-CoV-1. SHC014 had been isolated from bat guano found in caves in Yunnan province, which, as we mentioned above, is 802 miles from Wuhan and was the scene of an outbreak of coronavirus-associated respiratory disease among miners. The SHC014 had been donated by Shi Zhengli of the Wuhan Institute of Virology, who had been researching these caves in search of potential emerging human pathogens. The resulting genetically engineered viruses showed the ability to grow in cultured human lung airway tissue.
Baric, a specialist in coronaviruses, justified this experiment as a means of anticipating the nature of a potential emerging pathogen in the interest of finding a vaccine against it. In 2015 Baric published his paper “A SARS-like cluster of circulating bat coronaviruses shows potential for human emergence”.
Although Baric had insisted that because his experiments were carried out under the most stringent biosafety conditions (BSL-4) the risk was acceptable, the NIH placed a moratorium on GoF research in 2014, in the U.S.. It was at that time that the epicenter of this research shifted to China. The NIH approved $600,000 in grants to the Wuhan Institute of Virology from 2014 to 2018, and has consistently declined to release the details of these grants to the public.
Shi’s work focused on the Yunnan bat caves, which were in the general area where the SARS-CoV-1 outbreak took place, and her stated goal was to find coronaviruses resembling SARS-CoV-1 that had high potential for human transmission. Two strains that Zhi found in bat guano from these caves were SHC014 and WIV1; the latter already showed the ability to grow in cultured human airway cells, and provided the human-adaptive spike protein that was spliced with the already partially human-adapted and human-pathogenic SARS-CoV-1.
In 2014, the NIH awarded a five-year, $3.75 million grant to Peter Daszak’s EcoHealth Alliance to study the risk that novel bat-borne coronaviruses would emerge in China. The Wuhan Institute of Virology received a substantial share of this grant, which went into financing the research carried out there which we describe above. The New York based nongovernmental organization EcoHealth Alliance describes itself as dedicating to protecting people, animals, and the environment from emerging infectious diseases. However, most of the organization’s day-to-day funding comes from the Departments of Defense and Homeland Security. We have already mentioned that gain of function research is considered dual function—medical and military—and in fact the dual funding from the health bureaucracy and the war establishment points out the true nature of this sinister organization with a hippie-sounding name.
EcoHealth Alliance: Creepy Do-Gooders The EcoHealth Alliance also applied for a grant from the Defense Advanced Research Projects Agency (DARPA; originator of the Internet and the source of the seed money for the Moderna Covid-19 vaccine) for Project Defuse, a plan to create mutated bat coronaviruses that could infect humans. The EcoHealth Alliance proposed to directly vaccinate bats in caves in southern China against these artificially human-adapted bat viruses in order to PREVENT the next SARS outbreak. The mutation that they were talking about was the insertion of a site for cleavage of the furin protease in the spike protein of naturally occurring bat coronaviruses, which as we have explained above is the mechanism that enables SARS-CoV-2 to infect human airway cells, resulting in deadly Covid-19.
The official story is that this grant was denied by DARPA. However, we are presented with the plain facts that SARS-CoV-2, the agent of the pandemic that started in 2019, possesses a furin-cleavage site that is key to its infectivity.
You Make The Conclusions The above discussion can be summed up as follows:
1. SARS-CoV-2 emerged in the city of Wuhan, over 300 miles from the nearest concentration of coronavirus bearing bats, already fully adapted for human transmission.
2. It differs from all natural bat coronaviruses by possessing a furin-cleavage site, which enables the virus to interact with receptors in human airway cells.
3. The EcoHealth Alliance precisely proposed adding a furin-cleavage site to the natural coronavirus as a means of creating a human-adapted coronavirus, with the ostensible innocent aim of vaccinating cave bats in order to prevent a pandemic such as the one that actually took place.
4. It is not possible to dismiss the idea that irresponsible research was at the origin of the Covid-19 pandemic.
5. Although perhaps less likely, neither is it possible to dismiss the idea that irresponsible military research was at the origin of the pandemic, because these gain of function experiments are regarded as dual-use. |